Ultrapure media
Storage and distribution systems for ultrapure media
SCHRADER storage and distribution systems for ultrapure media
In addition to storage and distribution systems for supply media for e.g. cooling water or process steam, SCHRADER also supplies high-quality systems for the distribution and storage of:
Water for injections
(Water for Injection, WFI)
Distribution of pure steam and
return of
pure steam condensate
Pure water
(Purified Water, PW)
The design is customer-specific according to the respective requirements for the product and the sales market, as well as the resulting regulations and laws, so that product safety is guaranteed at all times. SCHRADER offers standard solutions for hot storage (65 – 85 °C) or cold storage (15 – 20 °C).
The distribution of the ultrapure media takes place in loops. In order to avoid contamination or the formation of biofilms, these systems are designed in such a way that a steady, turbulent flow (Re > 10,000) in pipelines and fittings is ensured. SCHRADER also offers systems with hot storage and cold sub-loops (“mixed systems”), while regular sanitisation is carried out either cold by ozonation or hot by heating over 85 C.
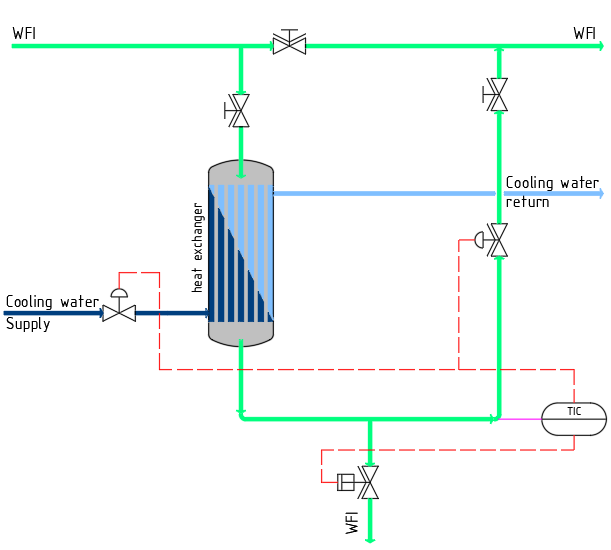
The graphic shows a section of a “mixed system” for the storage and distribution of WFI. The shown sub-loop allows the removal of both hot and cold WFI. The return of the sub-loop can be throttled during a cold removal, in order to avoid cooling of the main ring with small removal quantities.
The plant and piping systems are made of stainless steel and – as far as possible – orbital welded. The monitoring and the associated sensors can be individually adapted:
- Flow, temperature, pressure and conductivity measurement
- Determination of total organic carbon (TOC) in the medium
- Degree of automation
- Filling station management in various versions
- Interfaces:
- About existing systems
- Building Management Technology
- OPC Unified Architecture (OPC UA)
The execution is carried out in accordance with the regulations of the EU GMP Guideline Annex 11 “Computer-aided systems” and the “21 CFR Part 11 Electronic signatures, electronic records”. The software is categorised and validated according to GAMP5.
Contact
We are at your disposal by phone, e-mail and with a global network of experienced engineers worldwide. Just contact us.